Nature Reviews Microbiology Gum Disease Implant Catheter Contamination

Electrochemical Disinfection of Dental Implants Experimentally Contaminated with Microorganisms equally a Model for Periimplantitis
1
Microbiology Sectionalization, Department of Biological science, University of Erlangen-Nuremberg, 91058 Erlangen, Germany
2
Division of ultra-hard coatings, Department of Fabric Sciences, Academy of Erlangen-Nuremberg, 91058 Erlangen, Deutschland
3
Department of Prosthodontics, Saarland University, 66421 Homburg/Saar, Germany
iv
Chair of Materials Science and Engineering for Metals, Section of Material Sciences, University of Erlangen-Nuremberg, 91058 Erlangen, Federal republic of germany
*
Author to whom correspondence should be addressed.
Received: 17 January 2020 / Revised: three February 2020 / Accepted: 6 February 2020 / Published: 9 February 2020
Abstruse
Despite several methods having been described for disinfecting implants affected by periimplantitis, none of these are universally effective and may even alter surfaces and mechanical properties of implants. Boron-doped diamond (BDD) electrodes were made from niobium wires and assembled every bit a unmarried instrument for implant cleaning. Chemo-mechanical debridement and air abrasion were used every bit control methods. Different mono-species biofilms, formed by bacteria and yeasts, were immune to develop in rich medium at 37 °C for three days. In add-on, natural multi-species biofilms were treated. Implants were placed in silicone, polyurethane foam and bovine ribs for simulating different clinical weather condition. Following handling, the implants were rolled on claret agar plates, which were subsequently incubated at 37 °C and microbial growth was analyzed. Complete electrochemical disinfection of implant surfaces was achieved with a maximum treatment time of 20 min for Candida albicans, Candida dubliniensis, Enterococcus faecalis, Roseomonas mucosa, Staphylococcus epidermidis and Streptococcus sanguinis, while in case of spore-forming Bacillus pumilus and Bacillus subtilis, a number of colonies appeared afterwards BDD electrode treatment indicating an incomplete disinfection. Independent of the species tested, consummate disinfection was never achieved when conventional techniques were used. During treatment with BDD electrodes, only minor changes in temperature and pH value were observed. The musical instrument used here requires optimization so that higher charge quantities can exist applied in shorter handling times.
1. Introduction
While a consequent definition of periimplantitis [1] every bit well as diagnostic criteria defining this affliction are still missing [2,three], periimplantitis has received tremendous attention during the past years [3]. In addition, considerable heterogeneity exists amongst reports on the prevalence of periimplantitis [three]. A systematic review and meta-assay conducted by Dreyer and coworkers found prevalence on implant level between 1.one% and 85.0% [2], while Rakic and coworkers found prevalence of 12.viii% at implant level [four]. Irrespective of the exact numbers, periimplantitis remains beingness a complication, threatening long-term implant survival [5,6].
Co-ordinate to a recent literature review, periimplantitis has a multifactorial etiology [3] for which several risk factors [7,8] have been described. These include patient-specific factors such every bit genetic disorders, smoking [1] and periodontal disease [ix,10], cement or impression textile remnants in the periimplant sulcus [11,12], bacterial contamination of the implant components [thirteen], technical issues and implant surface characteristics [five,10,fourteen].
Several attempts already take been made aimed at characterizing periimplant lesions every bit well every bit differences between teeth and implants with respect to pathologic processes [15,16]. While the exact patho-mechanisms seem not to exist fully understood, information technology appears to exist consensus that bacterial biofilms on dental implants [13,17] can crusade an inflammatory reaction [12,xviii,19], resulting in loss of periimplant bone [20,21]. All the same, it has also been pointed out that marginal bone loss around dental implants may result from the bone'southward response to surgical trauma and implant loading which must be differentiated from periimplantitis [22].
Almost treatment strategies depend on the clinical state of affairs considering probing depth, suppuration, periodontal indices such as bleeding on probing and plaque index as well every bit radiographic bone loss [3,23,24,25] as decisive factors. Handling may then range from implant debridement, resective and reconstructive surgery [23] to implant removal [26]. According to several authors, handling of periimplantitis is considered as having an unpredictable outcome [6,xx] in particular when evaluating the effectiveness of regenerative treatment [23,27].
A broad variety of techniques for the disinfection of dental implants has been described in the literature [26]. These include mechanical instrumentation [28,29], chemic and antimicrobial agents [30], treatment with local or systemic antibiotics [17,29,31], laser application [32], photodynamic therapy [xviii,33,34], common cold plasma handling [35,36] and air abrasion [37,38,39,40]. In many instances, only combinations of different disinfection techniques have been shown to exist effective [36,40,41,42], with mechanical debridement bearing the hazard of implant surface alterations [43]. A novel and simply recently published arroyo employs electrochemical principles for in situ removal of biofilm from textured dental implant surfaces using the implant itself every bit an electrode [44].
In this preliminary proof of principle study, we tested the application of boron-doped diamond (BDD) electrodes [45,46,47] for the electrochemical disinfection of dental implants colonized past biofilm-forming microorganisms. The working principle of these electrodes is based on the electrolytic dissociation of h2o, which theoretically produces hydrogen at the negative pole (cathode) and oxygen at the positive pole (anode). Due to the properties of the electrode material used, a higher voltage than theoretically needed is required (overpotential). When diamond electrodes are being used, an overpotential of 2.8 V is required for anodic oxygen production while the desired disinfective OH radicals are generated already at 2.5 V.
2. Materials and Methods
ii.one. Preparation of Electrodes
Diamond coating with boron doping of thin niobium wires (200 µm in diameter) was performed in a Hot-Filament Chemical Vapor Deposition machine at approx. 800 °C in a methane-hydrogen-trimethylborate gas temper at 2 mbar for 6 h. The functionality and the manageability of the diamond coatings where verified using bending tests. Optimal properties with respect to stiffness and surface roughness were obtained when the niobium wires were sandblasted (air pressure 4 bar) with silicon-carbide particles approximately 46 µm in size prior to BDD coating. A special wire coating setup was developed to minimize deformation of the wires during diamond blanket. With this arroyo, a reproducible thickness of the dense diamond coating of approximately ii µm could be achieved (Effigy ane). Two of these electrodes were combined with electric insulating media to grade a probe-like instrument with clinically applicable dimensions (Figure 2). This probe was connected to an external electric power supply allowing for adjusting voltage and treatment time.
2.2. Treatment of Experimentally Contaminated Implants as Periimplantitis Model
Forty-v commercially available dental implants with a medium crude surface (Straumann Bone Level Tapered 4.ane x 12 mm RC; REF: 021.5512; LOT: RP027) were exposed to different microbes for 3 days at 37 °C in rich medium [Brain Eye Infusion (BHI); Oxoid, Wesel, Germany] to allow biofilm formation on external and internal surfaces (Annotation: Implants were reused following sterilization in an autoclave in gild to increase sample size; please cf. Table S1). The microorganisms practical included yeasts (Candida albicans, Candida dubliniensis), Gram-negative (Roseomonas mucosa) and Gram-positive bacteria (Enterococcus faecalis, Staphylococcus epidermidis, Streptococcus sanguinis), including spore-forming bacteria (Bacillus pumilus, Bacillus subtilis) (Tabular array 1). The microorganisms were chosen based on their robustness and occurrence in cases of infected root canals [31,32] as well equally in cases of peri-implantitis [33,34]. Often found members of the genera Prevotella and Treponema were not included in the study, since these anaerobic and microaerophilic leaner are highly oxygen-sensitive and less resistant against reactive oxygen species and fifty-fifty atmospheric oxygen concentrations. For command of biofilm germination, staining and quantitative analysis was carried out as described previously [48].
The implants were either placed in (i) elastic silicone, stable against temperature, acid, base of operations and oxidants and bacterial colonization (Bindulin, Fürth, Germany), mimicking periimplant soft tissue, polyurethane foam blocks (Cellular Rigid polyurethane foam 20pcf, Sawbones Europe AB, Malmö, Sweden) mimicking type IV alveolar bone (Effigy 3) according to the Lekholm and Zarb nomenclature [51] or in bovine ribs. In the latter cases, osteotomies were created in preformed saucer-shaped defects applying the regular surgical protocol [39]. The defects false round bone resorption under maintenance of the buccal and oral compacta resembling class Ie defects co-ordinate to a clinical classification system [52]. These specimens were placed in containers filled with phosphate-buffered saline (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPOfour, ii mM KH2POfour, pH vii.4) and treated applying the post-obit methods: (i) Mechanical debridement (stainless steel curettes (EXD11/12, HuFriedy, Chicago, IL, USA), polishing of accessible implant surfaces) and irrigation with chlorhexidine (Chlorhexamed FORTE ethanol-free 0.2%, GlaxoSmithKline Consumer Healthcare GmbH & Co. KG, Munich, Germany) for a full of 5 min. (ii) Air abrasion (AIRFLOW PLUS, EMS ElectroMedicalSystems GmbH, Munich, Frg) and irrigation with chlorhexidine (Chlorhexamed FORTE ethanol-complimentary 0.2%, GlaxoSmithKline Consumer Healthcare GmbH & Co. KG) for a total of 5 min. (iii) Electrochemical disinfection using the electrode configuration described above applying unlike treatment times. For every species investigated, at least 3 biological replicates were tested for each treatment procedure.
Subsequently cleaning and disinfection, the implants were rolled five to 7 times on Columbia Blood Agar plates (Oxoid, Wesel, Frg), which were subsequently incubated at 37 °C for one day. Bacterial growth was monitored and rated using an evaluation scheme adjusted from a monitoring scheme of catheter infections [53]. To this stop, each lane of the curl-out was rated from 0 (no growth) to 3 (strong growth) using a master sample as reference (Figure 4). All experiments were carried out in contained replicates (n = 3 biological replicates).
ii.iii. Statistical Analysis
The ratings obtained for mechanical debridement, air chafe and v min BDD electrode handling of implants (n = 3) covered by E. faecalis, C. dubliniensis and multi-species biofilm were bailiwick to comparative statistical analysis. To this end, the sums of the individual ratings were used. In view of the modest sample sizes, the integer valued results cannot exist causeless to be normally distributed. Therefore, the parameter-free Kruskal Wallis rank sum examination (R, version iii.half-dozen.2, The R Foundation for Statistical Computing, Vienna, Republic of austria; www.R-project.org) was practical to compare the different treatments. The level of significance was set at α = 0.05.
three. Results
3.one. Biofilm Formation on Experimetally Contaminated Implants
Information technology was the aim of this report, to investigate the elimination of biofilms on implants using BDD electrodes every bit new electrochemical treatment method for periimplantitis. Equally a prerequisite of our experiments, implants were incubated in rich medium with distinct microbial species for several days. Later, biofilm formation was tested by staining with crystal violet solution. In all cases, biofilm formation on implants was observed; notwithstanding, in a species-specific amount. The strongest mono-species biofilm producers were B. subtilis and C. dubliniensis, moderate amounts of biofilm were produced by B. pumilus, R. mucosa and Due south. sanguinis, while the poorest colonization of implants tested hither was observed for C. albicans, Eastward. faecalis and South. epidermidis (Figure 5).
3.2. Removal of Biofilm from Implants Contaminated with C. dubliniensis
BDD application to implants placed in both, silicone and polyurethane foam for 10 min at constant 6 Five was at to the lowest degree as effective equally the control treatments i.e. mechanical debridement and air chafe. Increasing the treatment times of BDD electrodes led to even better results (Figure vi).
3.three. Removal of Biofilm from Implants Contaminated with Eastward. faecalis
Mechanical debridement and air abrasion were non suitable for consummate emptying of E. faecalis biofilm and disinfection of the implants (Figure 7). In contrast, using BDD electrode treatment, full removal of biofilm and complete disinfection of implants was achieved within v to 10 min depending on the model system used (Figure 7).
iii.4. Removal of Multispecies Biofilms from Implants
When bovine ribs were used to insert implants, a multi-species colonization was observed, despite the fact that only Due east. faecalis was used for pre-incubation and colonization. Apparently, the tested os material was already strongly contaminated with a number of dissimilar microorganisms and, consequently, a natural multi-species biofilm developed in the prepared bone. Handling of these implants showed an inferior disinfection success. This result may be explained by the putative presence of spore-forming bacteria in the uncharacterized natural multi-species biofilm and the fact that bone droppings, which was not removed prior to rolling the specimens on the claret agar plates, was sticking on the implant surface. All the same, compared to mechanical debridement and air chafe, BDD treatment still performed best (Figure 8).
3.5. Statistical Comparing Between Treatment Methods
The hateful values of the ratings every bit well every bit the results of the Kruskal Wallis rank sum tests for comparing the three different treatment modalities are given in Table 2. In no instance, a statistically significant difference could be observed betwixt the disinfection techniques applied.
iii.6. Fourth dimension-Dependent Removal of Biofilm from Implants Contaminated with Different Microorganisms
In addition to the microorganisms mentioned above and multi-species biofilm, the consequence of BDD electrode handling on mono-species biofilms formed past a number of different bacteria and yeasts was tested. The fastest elimination (within ten min) was achieved for E. faecalis, R. mucosa, Southward. sanguinis and C. dubliniensis. A slightly longer treatment time of 20 min was necessary for the disinfection of implants colonized past C. albicans and Due south. epidermidis. In case of spore-forming B. pumilus and B. subtilis, a significant reduction of growth but no complete disinfection was reached nether the experimental conditions practical due to the formation of highly resistant spores (six 5, v–22 mA) (Table 3).
3.7. Influence of BDD Electrode Treatment on Temperature and pH
Successful treatment of periimplantitis must not simply rely on a reproducible disinfection protocol, but also on the absence of negative side furnishings. Therefore, changes in physico-chemic parameters depending on BDD treatment were tested. Afterward treatment times of 25 min, changes in temperature in the range of two °C and changes in pH of i unit were recorded in a controlled setting with a reaction tube filled with 4 ml of phosphate-buffered saline and the respective probes mounted in the straight surrounding of the electrodes (Figure 9).
4. Word
Despite using a simplistic prototype, it was shown that electrochemical disinfection of contaminated implant surfaces was possible when BDD electrodes based on niobium wires were used. In contrast to mechanical debridement damaging the implant surface and air pulverization abrasion leaving pulverization remnants on the implant surface, no alterations of the implant surfaces were identified with the employ of BDD electrodes [54]. Every bit expected, the different microbes tested in this study showed varying levels of sensitivity and hence required varying amounts of treatment time until complete disinfection was achieved. The worst performer in this respect were, besides an uncharacterized multi-species biofilm, B. pumilus and B. subtilis, both forming highly resistant spores under the experimental weather condition applied, while E. faecalis, R. mucosa and S. sanguinis were safely eliminated with a maximum handling time of 10 min. With the not-optimized setup used hither, these treatment times would be too long for clinical application. The disquisitional aspect, however, is not the treatment fourth dimension but the charge quantity applied. Time to come developments will hence include modified electrodes with increased surfaces.
Minor temperature and pH-value changes were observed after applying the electrodes for approximately 25 min and hence would not be expected in a clinical setting where clearly shorter treatment times are needed. Even if these changes would occur, negative furnishings on the patient cannot be expected.
Information technology had been predictable that disinfection would get more difficult when implants are placed in bone surrogate materials or cadaver bone equally compared to having total access to all critical surfaces. While non expressible equally a quantitative effect, this correlation was observed for the control treatments of chemo-mechanical debridement and air abrasion, respectively. In contrast, when electrochemical disinfection was applied, restricted access to implant surfaces patently had less effect.
Recently, an electro-chemical arroyo for implant debridement and periimplantitis handling was introduced utilizing the implant itself every bit an electrode (cathode) while a wire, not contacting the infected surface, was used as another electrode (anode) [44]. By this approach, not using BDD-coated electrodes, biofilm appears to be primarily removed past bubbling of electrochemically generated hydrogen at the cathodic implant surface. This theoretically bears the take a chance of spreading the biofilm into the surrounding tissue and may also generate corrosion problems on the implant surface by hindering the cocky-passivation of titanium subsequently damaging the native oxide layer by a mechanical load. Furthermore, the hydrogen uptake of the titanium implant can lower the mechanical strength of the material past hydrogen embrittlement [55].
Given that the experiment at hand constituted the starting time application of BDD electrodes for biofilm removal from dental implant surfaces, a number of limitations accept to be taken into account when interpreting the findings presented. A wide diversity of leaner and yeasts have been shown to be nowadays in dental biofilms, while in the current experiment by and large mono-species biofilms were considered. Although dissimilar bacteria may form a synergistic biofilm, their resistance to disinfecting measures is not influenced by other species. Even so, with the spore-forming species used hither, a worst-case scenario has already been tested. It has been shown that not only the clinical state of affairs per se but likewise the defect morphology impacts the result of periimplantitis handling [52]. As such, the model situations chosen here clearly present simplifications of reality, which were needed due to the prototypical stage of the BDD electrode setup. Due to the exploratory nature of this airplane pilot investigation, comparative statistical analysis amid the treatment modalities applied was express to a subset of experiments where implants had been treated for exactly 5 min using all three disinfection methods. Future studies are under mode with much greater sample size aimed at quantitatively comparison different handling modalities.
Taking into account that numerous developmental steps including preclinical and clinical studies will be required prior to clinical application of an instrument based on a BDD electrode assortment, a probe-like instrument with a permeable cover can already be envisaged. Non requiring superstructure removal as well as the universal applicability besides in periodontal and endodontic handling would be major advantages of such an instrument.
5. Patents
Stefan Rosiwal, Andreas Burkovski, Maximilian Göltz and Matthias Karl have a filed a patent for the disinfection method described in this report.
Supplementary Materials
The following are available online at https://world wide web.mdpi.com/2077-0383/9/2/475/s1, Table S1: Overview of disinfection experiments. Sample size and groups are given for organisms and treatment methods applied (due north.d., not adamant). Embedment methods used: silicone, polyurethane foam and bovine ribs.
Author Contributions
A.B., M.Thou. (Matthias Karl) and South.R. were involved in conceptualization of the study, G.Thou. carried out diamond coating of niobium wires, M.Thou. (Maximilian Koch) designed the protoype electrode, One thousand.Thou. (Maximilian Koch) and Yard.10. carried out respective experiments. A.B. supervised the microbiology work, S.R. the diamond-coating process. Writing and original draft preparation was carried out by A.B., M.Thousand. (Matthias Karl) and Due south.R., A.B. and M.Thou. (Matthias Karl) finalized the manuscript. M.1000. (Matthias Karl) was involved in funding conquering. All authors accept read and agreed to the published version of the manuscript.
Funding
This project was supported by a grant (1328_2018) from the ITI Foundation, Switzerland.
Acknowledgments
We acknowledge support past the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) and Saarland University inside the funding programme Open up Access Publishing. The authors wish to thank Friedrich Graef, Department of Mathematics, University of Erlangen-Nuremberg for statistical data analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pimentel, S.P.; Shiota, R.; Cirano, F.R.; Casarin, R.C.V.; Pecorari, Five.Chiliad.A.; Casati, M.Z.; Haas, A.Due north.; Ribeiro, F.V. Occurrence of peri-implant diseases and risk indicators at the patient and implant levels: A multi-level cross-exclusive study. J. Periodontol. 2018, 89, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, H.; Grischke, J.; Tiede, C.; Eberhard, J.; Schweitzer, A.; Toikkanen, S.East.; Glöckner, S.; Krause, Thou.; Stiesch, M. Epidemiology and risk factors of peri-implantitis: A systematic review. J. Periodontal. Res. 2018, 53, 657–681. [Google Scholar] [CrossRef] [PubMed]
- Lin, Thou.H.; Kapila, Y.; Wang, H.L. Parameters to define peri-implantitis: A review and a proposed multi-domain scale. J. Oral Implantol. 2017, 43, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Rakic, Yard.; Galindo-Moreno, P.; Monje, A.; Radovanovic, South.; Wang, H.L.; Cochran, D.; Sculean, A.; Canullo, L. How frequent does peri-implantitis occur? A systematic review and meta-assay. Clin. Oral Invest. 2018, 22, 1805–1816. [Google Scholar] [CrossRef]
- Derks, J.; Schaller, D.; Håkansson, J.; Wennström, J.Fifty.; Tomasi, C.; Berglundh, T. Effectiveness of implant therapy analyzed in a Swedish population: Prevalence of peri-implantitis. J. Dental Res. 2016, 95, 43–49. [Google Scholar] [CrossRef]
- Kane, G.; Fang, V.; Simon, S.; Girdler, J.; Adeyinka, O.; Alhilou, A. A survey exploring the experiences & attitudes of dental implant clinicians in the direction of peri-implantitis inside the Britain. Eur. J. Prosthodont. Restor. Dent. 2018, 26, 46–52. [Google Scholar] [CrossRef]
- Albrektsson, T.; Buser, D.; Sennerby, L. Crestal bone loss and oral implants. Clin. Implant Dent. Relat. Res. 2012, 14, 783–791. [Google Scholar] [CrossRef]
- Albrektsson, T.; Canullo, L.; Cochran, D.; De Bruyn, H. "Peri-implantitis": A complication of a foreign body or a human-made "disease". Facts and fiction. Clin. Impl. Dent. Relat. Res. 2016, 18, 840–849. [Google Scholar] [CrossRef]
- Kumar, P.Southward.; Dabdoub, S.Chiliad.; Hegde, R.; Ranganathan, N.; Mariotti, A. Site-level risk predictors of peri-implantitis: A retrospective analysis. J. Clin. Periodontol. 2018, 45, 597–604. [Google Scholar] [CrossRef]
- Derks, J.; Håkansson, J.; Wennström, J.Fifty.; Tomasi, C.; Larsson, M.; Berglundh, T. Effectiveness of implant therapy analyzed in a Swedish population: Early and belatedly implant loss. J. Dent. Res. 2015, 94, 44S–51S. [Google Scholar] [CrossRef]
- Korsch, Chiliad.; Robra, B.P.; Walther, W. Cement-associated signs of inflammation: Retrospective analysis of the effect of excess cement on peri-implant tissue. Int. J. Prosthodont. 2015, 28, eleven–18. [Google Scholar] [CrossRef] [PubMed]
- Pesce, P.; Canullo, L.; Grusovin, M.G.; de Bruyn, H.; Cosyn, J.; Pera, P. Systematic review of some prosthetic hazard factors for periimplantitis. J. Prosthet. Dent. 2015, 114, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Al-Ahmad, A.; Muzafferiy, F.; Anderson, A.C.; Wölber, J.P.; Ratka-Krüger, P.; Fretwurst, T.; Nelson, K.; Vach, K.; Hellwig, E. Shift of microbial limerick of peri-implantitis-associated oral biofilm every bit revealed by 16S rRNA gene cloning. J. Med. Microbiol. 2018, 67, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Gallego, L.; Sicilia, A.; Sicilia, P.; Mallo, C.; Cuesta, South.; Sanz, Thou. A retrospective report on the crestal bone loss associated with unlike implant surfaces in chronic periodontitis patients under maintenance. Clin. Oral Implants Res. 2018, 29, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Eggert, F.Thousand.; Levin, 50. Biology of teeth and implants: Host factors - pathology, regeneration, and the role of stem cells. Quintessence Int. 2018, 49, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Bastos, M.F.; de Franco, L.; Garcia Tebar, A.C.; Giro, G.; Shibli, J.A. Expression levels of Semaphorins 3A, 3B, 4A, and 4D on human peri-implantitis. Int. J. Oral Maxillofac. Implants 2018, 33, 565–570. [Google Scholar] [CrossRef]
- Patianna, G.; Valente, N.A.; D'Addona, A.; Andreana, S. In vitro evaluation of controlled-release xiv% doxycycline gel for decontamination of machined and sandblasted acid-etched implants. J. Periodontol. 2018, 89, 325–330. [Google Scholar] [CrossRef]
- Bombeccari, G.P.; Guzzi, Grand.; Gualini, F.; Gualini, S.; Santoro, F.; Spadari, F. Photodynamic therapy to treat periimplantitis. Implant Dent. 2013, 22, 631–638. [Google Scholar] [CrossRef]
- Kasnak, G.; Firatli, E.; Könönen, E.; Olgac, V.; Zeidán-Chuliá, F.; Gursoy, U.K. Elevated levels of 8-OHdG and PARK7/DJ-1 in peri-implantitis mucosa. Clin. Implant Dent. Relat. Res. 2018, 20, 574–582. [Google Scholar] [CrossRef]
- Wong, R.L.; Hiyari, Due south.; Yaghsezian, A.; Davar, Grand.; Casarin, M.; Lin, Y.L.; Tetradis, Southward.; Camargo, P.M.; Pirih, F.Q. Early intervention of peri-implantitis and periodontitis utilizing a mouse model. J. Periodontol. 2018, 89, 669–679. [Google Scholar] [CrossRef]
- Berglundh, T.; Armitage, Grand.; Araujo, Thou.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, East. Peri-implant diseases and conditions: Consensus report of workgroup four of the 2017 World Workshop on the Nomenclature of Periodontal and Peri-Implant Diseases and Atmospheric condition. J. Clin. Periodontol 2018, 45 (Suppl. 20), S286–S291. [Google Scholar] [CrossRef]
- Albrektsson, T.; Chrcanovic, B.; Östman, P.O.; Sennerby, 50. Initial and long-term crestal bone responses to modernistic dental implants. Periodontol. 2000 2017, 73, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Mercado, F.; Hamlet, S.; Ivanovski, S. Regenerative surgical therapy for peri-implantitis using deproteinized bovine bone mineral with x% collagen, enamel matrix derivative and Doxycycline-A prospective 3-year cohort study. Clin. Oral Implants. Res. 2018, 29, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Monje, A.; Caballé-Serrano, J.; Nart, J.; Peñarrocha, D.; Wang, H.L.; Rakic, 1000. Diagnostic accuracy of clinical parameters to monitor peri-implant conditions: A matched case-control study. J. Periodontol. 2018, 89, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Ramanauskaite, A.; Becker, Chiliad.; Schwarz, F. Clinical characteristics of peri-implant mucositis and peri-implantitis. Clin. Oral Implants Res. 2018, 29, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Sinjab, G.; Garaicoa-Pazmino, C.; Wang, H.Fifty. Conclusion making for management of periimplant diseases. Implant Paring. 2018, 27, 276–281. [Google Scholar] [CrossRef]
- Isehed, C.; Svenson, B.; Lundberg, P.; Holmlund, A. Surgical treatment of peri-implantitis using enamel matrix derivative, an RCT: iii- and five-year follow-up. J. Clin. Periodontol. 2018, 45, 744–753. [Google Scholar] [CrossRef]
- Berglundh, T.; Wennström, J.Fifty.; Lindhe, J. Long-term upshot of surgical handling of peri-implantitis. A 2-11-year retrospective study. Clin. Oral Implants Res. 2018, 29, 404–410. [Google Scholar] [CrossRef]
- Gershenfeld, L.; Kalos, A.; Whittle, T.; Yeung, S. Randomised clinical trial of the furnishings of azithromycin use in the treatment of peri-implantitis. Aust. Paring. J. 2018. [Google Scholar] [CrossRef]
- Ribeiro, F.Five.; Casati, M.Z.; Casarin, R.C.; Corrêa, M.G.; Cirano, F.R.; Negri, B.M.; Pimentel, S.P. Impact of a triclosan-containing toothpaste during the progression of experimental peri-implant mucositis: Clincal parameters and local pattern of osteo-immunoinflammatory mediators in peri-implant fluid. J. Periodontol. 2018, 89, 203–212. [Google Scholar] [CrossRef]
- Carcuac, O.; Derks, J.; Abrahamsson, I.; Wennström, J.50.; Petzold, Thou.; Berglundh, T. Surgical handling of peri-implantitis: iii-year results from a randomized controlled clinical trial. J. Clin. Periodontol. 2017, 44, 1294–1303. [Google Scholar] [CrossRef] [PubMed]
- Takagi, T.; Aoki, A.; Ichinose, S.; Taniguchi, Y.; Tachikawa, N.; Shinoki, T.; Meinzer, W.; Sculean, A.; Izumi, Y. Effective removal of calcified deposits on microstructured titanium fixture surfaces of dental implants with erbium lasers. J. Periodontol. 2018, 89, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Ramos, U.D.; Suaid, F.; Wikesjö, U.M.; Susin, C.; Vital, P.C.; de Souza, S.50.Southward.; Messora, Chiliad.R.; Palioto, D.B.; Novaes, A.B., Jr. Microbiologic issue of two topical anti-infective treatments on ligature-induced peri-implantitis: A airplane pilot written report in dogs. J. Periodontol. 2018, 89, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Fraga, R.Due south.; Antunes, L.A.A.; Fontes, G.B.F.D.C.; Küchler, Due east.C.; Iorio, Due north.L.P.P.; Antunes, L.S. Is antimicrobial photodynamic therapy constructive for microbial load reduction in peri-implantitis treatment? A systematic review and meta-analysis. Photochem. Photobiol. 2018, 94, 752–759. [Google Scholar] [CrossRef]
- Rupf, S.; Idlibi, A.N.; Marrawi, F.A.; Hannig, M.; Schubert, A.; von Mueller, L.; Spitzer, W.; Holtmann, H.; Lehmann, A.; Rueppell, A.; et al. Removing biofilms from microstructured titanium ex vivo: A novel approach using atmospheric plasma technology. PLoS. ONE 2011, 6, e25893. [Google Scholar] [CrossRef]
- Idlibi, A.N.; Al-Marrawi, F.; Hannig, G.; Lehmann, A.; Rueppell, A.; Schindler, A.; Jentsch, H.; Rupf, S. Destruction of oral biofilms formed in situ on machined titanium (Ti) surfaces by cold atmospheric plasma. Biofouling 2013, 29, 369–379. [Google Scholar] [CrossRef]
- Lee, S.T.; Subu, M.K.; Kwon, T.Thousand. Emphysema following air-powder annoying handling for peri-implantitis. Maxillofac. Plast Reconstr. Surg. 2018, 40, 12. [Google Scholar] [CrossRef]
- Tastepe, C.S.; Lin, X.; Werner, A.; Donnet, M.; Wismeijer, D.; Liu, Y. Cleaning result of osteoconductive powder abrasive treatment on explanted human implants and biofilm-coated titanium discs. Clin. Exp. Dent. Res. 2018, four, 25–34. [Google Scholar] [CrossRef]
- Wei, Yard.C.T.; Tran, C.; Meredith, North.; Walsh, L.J. Effectiveness of implant surface debridement using particle beams at differing air pressures. Clin. Exp. Dent. Res. 2017, 3, 148–153. [Google Scholar] [CrossRef]
- Rosen, P.S.; Qari, Grand.; Froum, S.J.; Dibart, S.; Chou, L.Fifty. A pilot study on the efficacy of a treatment algorithm to detoxify dental implant surfaces affected by peri-implantitis. Int. J. Periodontics Restorative Dent. 2018, 38, 261–267. [Google Scholar] [CrossRef]
- Stein, J.M.; Hammächer, C.; Michael, S.S. Combination of ultrasonic decontamination, soft tissue curettage, and submucosal air polishing with povidone-iodine application for not-surgical therapy of peri-implantitis: 12 Month clinical outcomes. J. Periodontol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Madi, 1000.; Htet, M.; Zakaria, O.; Alagl, A.; Kasugai, Due south. Re-osseointegration of dental implants after periimplantitis treatments: A systematic review. Implant Paring. 2018, 27, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Larsen, O.I.; Enersen, K.; Kristoffersen, A.K.; Wennerberg, A.; Bunæs, D.F.; Lie, S.A.; Leknes, K.N. Antimicrobial furnishings of three different treatment modalities on dental implant surfaces. J. Oral Implantol. 2017, 43, 429–436. [Google Scholar] [CrossRef]
- Schneider, S.; Rudolph, M.; Bause, V.; Terfort, A. Electrochemical removal of biofilms from titanium dental implant surfaces. Bioelectrochemistry 2018, 121, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Kraft, A. Doped diamond: A meaty review on a new, versatile electrode material. Int. J. Electrochem. Sci. 2007, 2, 355–385. [Google Scholar]
- Schorr, B.; Ghanem, H.; Rosiwal, S.; Geißdörfer, Due west.; Burkovski, A. Elimination of bacterial contaminations by treatment of water with boron-doped diamond electrodes. World J. Microbiol. Biotechnol. 2019, 35, 48. [Google Scholar] [CrossRef]
- Böhm, A.-L.; Koch, M.; Rosiwal, S.; Burkovski, A.; Karl, M. Grobecker-Karl, T. Electrochemical disinfection of experimentally infected teeth by boron-doped diamond electrode treatment. J. Clin. Med. 2019, eight, 2037. [Google Scholar] [CrossRef]
- O'Toole, Thousand.A.; Kolter, R. Initiation of biofilm germination in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signaling pathways: A genetic analysis. Mol. Microbiol. 1998, 28, 449–461. [Google Scholar] [CrossRef]
- Burkovski, A.; Karl, Grand. Lack of testify for the necessity of root culvert obturation. Quintessence Int. 2019, l, 2–eight. [Google Scholar]
- Diesendorf, Due north.; Köhler, S.; Geißdörfer, W.; Grobecker-Karl, T.; Karl, One thousand.; Burkovski, A. Characterisation of Roseomonas mucosa isolated from the root canal of an infected tooth. BMC. Res. Notes 2017, x, 212. [Google Scholar] [CrossRef]
- Lekholm, U.; Zarb, Thou.A. Patient selection and training. In Tissue Integrated Prostheses: Osseointegration in Clinical Dentistry; Branemark, P.-I., Zarb, G.A., Albrektsson, T., Eds.; Quintessence Publishing: Chicago, IL, United states of america, 1985; pp. 199–209. [Google Scholar]
- Schwarz, F.; Sahm, Northward.; Schwarz, K.; Becker, J. Affect of defect configuration on the clinical outcome following surgical regenerative therapy of peri-implantitis. J. Clin. Periodontol. 2010, 37, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Maki, D.G.; Weise, C.E.; Sarafin, H.W. A semiquantitative culture method for identifying intravenous-catheter-related infection. Northward. Engl. J. Med. 1977, 296, 1305–1309. [Google Scholar] [CrossRef] [PubMed]
- Göltz, M.; Koch, M.; Detsch, R.; Karl, M.; Burkovski, A.; Rosiwal, S. Influence of in-situ electrochemical oxidation on implant surface and colonizing microorganisms evaluated by scatter electron microscopy. Materials 2019, 12, 3977. [Google Scholar] [CrossRef]
- Tal-Gutelmacher, East.; Eliezer, D. The hydrogen embrittlement of titanium-based alloys. J. Min. Met. Mat. Soc. 2005, 9, 46–49. [Google Scholar] [CrossRef]
Effigy 1. Scanning electron microscopy (SEM) image of a niobium wire later boron doped diamond (BDD) coating (Right: Overview; Left: Close up view of the surface area indicated past black rectangle). The coating layer has an estimate thickness of 2 µm and shows no delamination.
Figure 1. Scanning electron microscopy (SEM) image of a niobium wire after boron doped diamond (BDD) blanket (Right: Overview; Left: Shut upwardly view of the area indicated by black rectangle). The coating layer has an approximate thickness of 2 µm and shows no delamination.
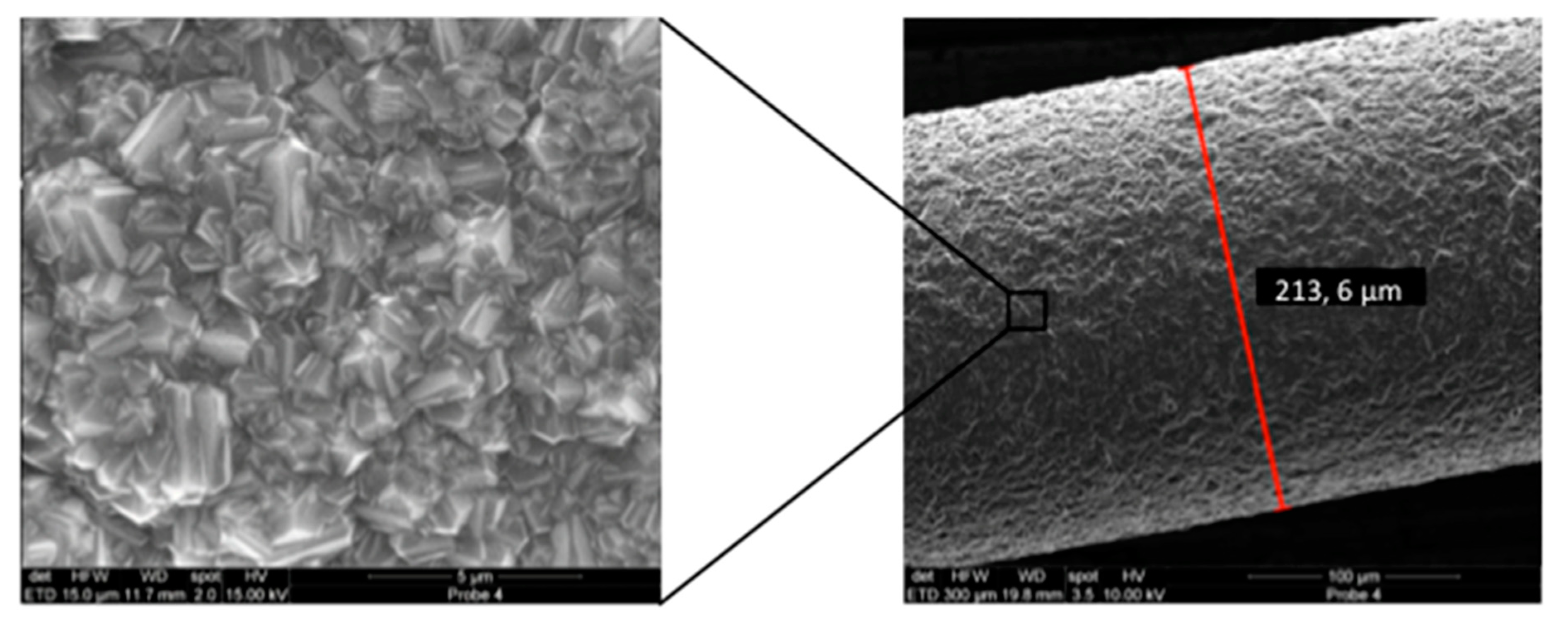
Figure ii. Disinfection apparatus consisting of an electrode assortment with intermediate insulating material.
Figure 2. Disinfection apparatus consisting of an electrode array with intermediate insulating cloth.

Figure 3. Placement of dental implants in model substrates. (A) Silicone simulating periimplant soft tissue, (B) polyurethane foam mimicking type IV alveolar bone with a circumferential defect and (C) bovine ribs.
Figure three. Placement of dental implants in model substrates. (A) Silicone simulating periimplant soft tissue, (B) polyurethane cream mimicking blazon 4 alveolar os with a circumferential defect and (C) bovine ribs.
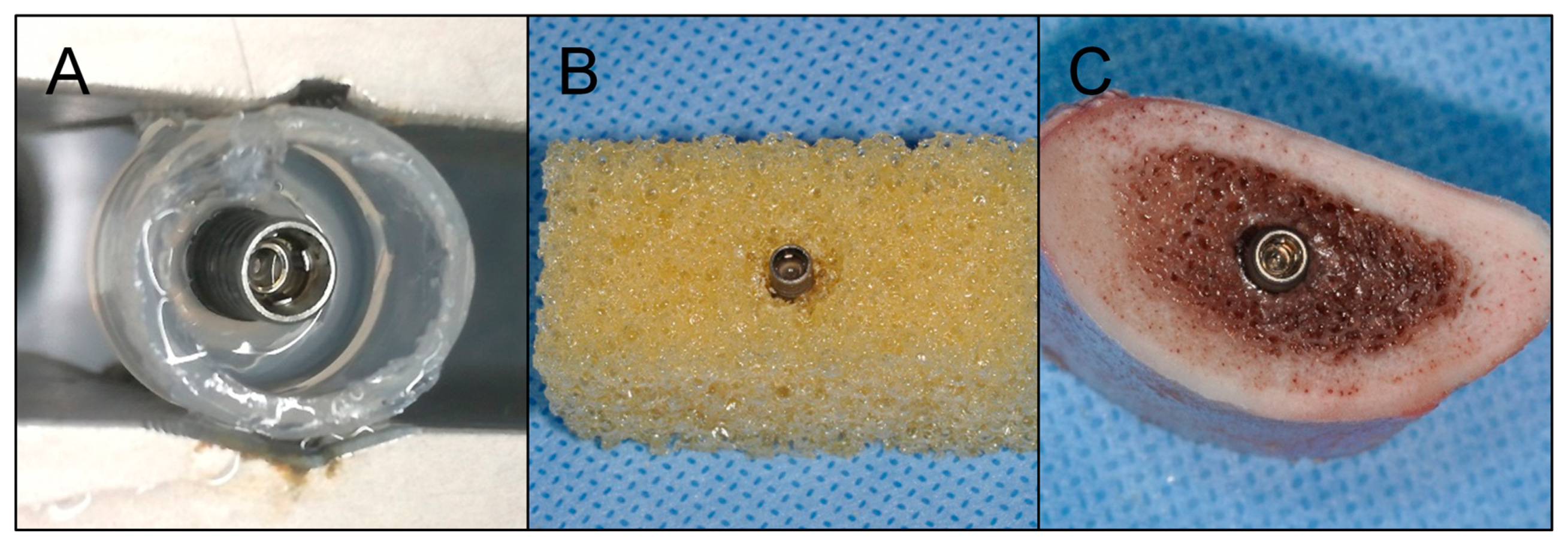
Figure 4. Evaluation scheme for microbial growth. Treated implants were rolled on Columbia Blood Agar plates and incubated at 37 °C. Growth of the individual roll-outs was scored from 0 = no bacterial growth, 1 = minor growth, 2 = major growth, 3 = stiff growth.
Figure 4. Evaluation scheme for microbial growth. Treated implants were rolled on Columbia Blood Agar plates and incubated at 37 °C. Growth of the private roll-outs was scored from 0 = no bacterial growth, 1 = minor growth, two = major growth, 3 = strong growth.

Figure 5. Biofilm germination on implants. Quantitative assay of biofilm germination: (1) B. pumilus, (2) B. subtilis, (3) C. albicans, (4) C. dubliniensis, (v) E. faecalis, (6) R. mucosa, (7) S. epidermidis, (8) S. sanguinis. Inset: Crystal violet staining of R. mucosa biofilm.
Effigy 5. Biofilm formation on implants. Quantitative assay of biofilm formation: (1) B. pumilus, (2) B. subtilis, (3) C. albicans, (4) C. dubliniensis, (five) Eastward. faecalis, (6) R. mucosa, (seven) S. epidermidis, (viii) S. sanguinis. Inset: Crystal violet staining of R. mucosa biofilm.
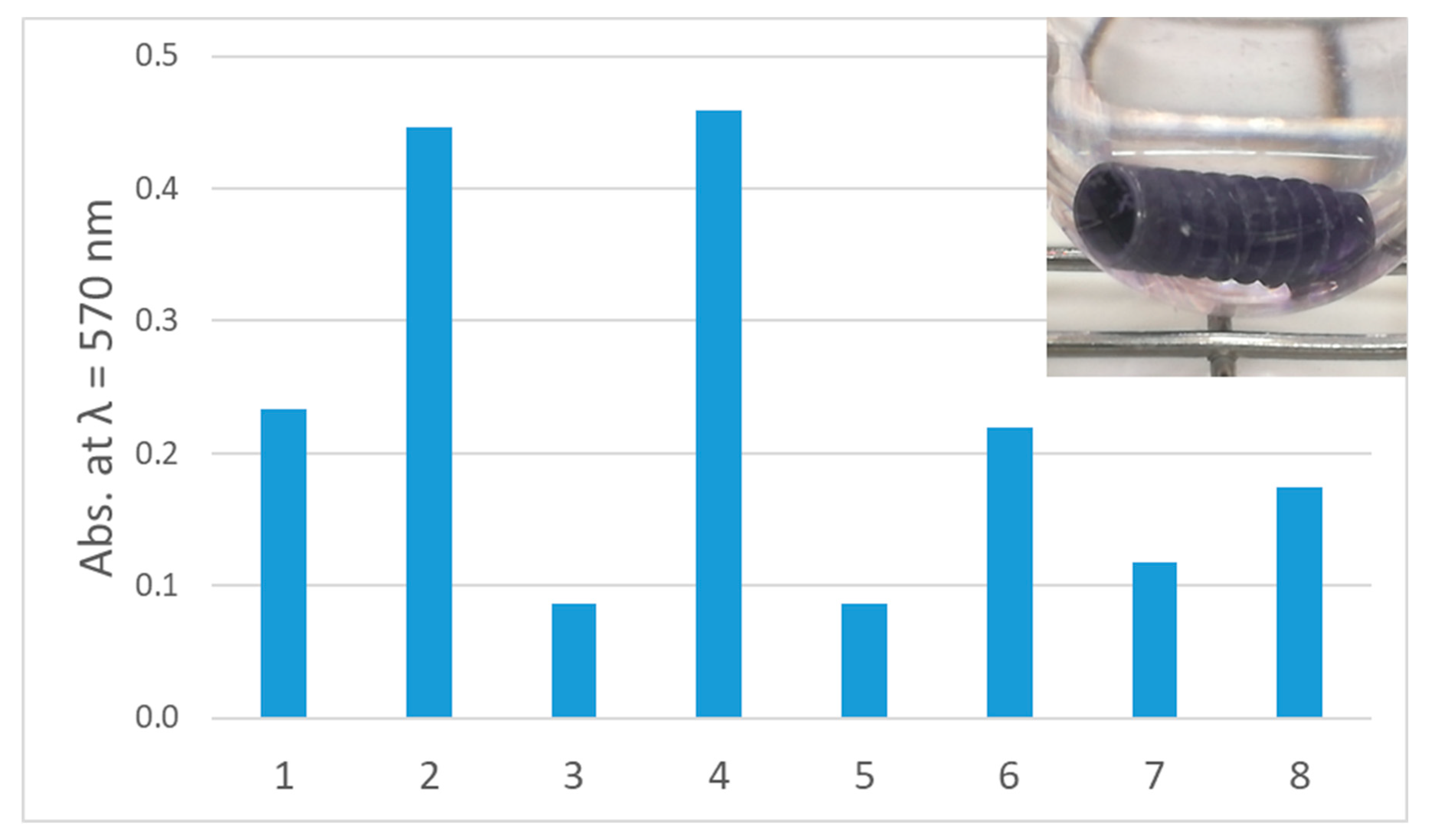
Figure 6. Treatment of C. dubliniensis biofilms. Upper panel: Comparison of growth on Columbia Blood Agar plates subsequently different treatment of implants infected with C. dubliniensis and placed in silicone. Lower panel: Quantitative comparison of growth on implants inserted in silicone (blueish bars) and polyurethane foam (orangish) depending on handling protocols. (A) Mechanical debridement, (B) air abrasion, (C) BDD treatment for 0 min, (D) BDD treatment for v min, (E) BDD treatment for 10 min, (F) BDD treatment for 15 min (north = 3 for each methods and time point). Growth of implant roll-outs was rated from 0 (no growth) to three (stiff growth). Columns represent the hateful of 3 independent biological replicated ± standard deviation (SD).
Figure 6. Treatment of C. dubliniensis biofilms. Upper panel: Comparison of growth on Columbia Blood Agar plates after dissimilar treatment of implants infected with C. dubliniensis and placed in silicone. Lower console: Quantitative comparison of growth on implants inserted in silicone (blue confined) and polyurethane foam (orangish) depending on treatment protocols. (A) Mechanical debridement, (B) air chafe, (C) BDD treatment for 0 min, (D) BDD handling for 5 min, (East) BDD treatment for 10 min, (F) BDD treatment for 15 min (n = 3 for each methods and fourth dimension point). Growth of implant ringlet-outs was rated from 0 (no growth) to 3 (strong growth). Columns represent the hateful of 3 independent biological replicated ± standard difference (SD).

Figure 7. Treatment of Eastward. faecalis biofilms. Upper console: Comparing of growth on Columbia Blood Agar plates after unlike treatment of implants infected with Eastward. faecalis and placed in silicone. Lower panel: Quantitative comparing of growth on implants inserted in silicone (blue bars) and polyurethane foam (orangish confined) depending on treatment protocols. (A) Mechanical debridement, (B) air abrasion, (C) BDD treatment for 0 min, (D) BDD handling for 5 min, (Eastward) BDD treatment for 10 min, (F) BDD treatment for xv min (north = 3 for each methods and time point). Growth of implant whorl-outs was rated from 0 (no growth) to 3 (strong growth). Columns represent the hateful of 3 independent biological replicated ± standard deviation (SD).
Figure 7. Handling of E. faecalis biofilms. Upper panel: Comparison of growth on Columbia Claret Agar plates later dissimilar treatment of implants infected with E. faecalis and placed in silicone. Lower panel: Quantitative comparison of growth on implants inserted in silicone (blue bars) and polyurethane foam (orangish bars) depending on treatment protocols. (A) Mechanical debridement, (B) air abrasion, (C) BDD treatment for 0 min, (D) BDD handling for 5 min, (E) BDD treatment for 10 min, (F) BDD treatment for fifteen min (n = 3 for each methods and time point). Growth of implant roll-outs was rated from 0 (no growth) to 3 (strong growth). Columns represent the mean of three independent biological replicated ± standard deviation (SD).
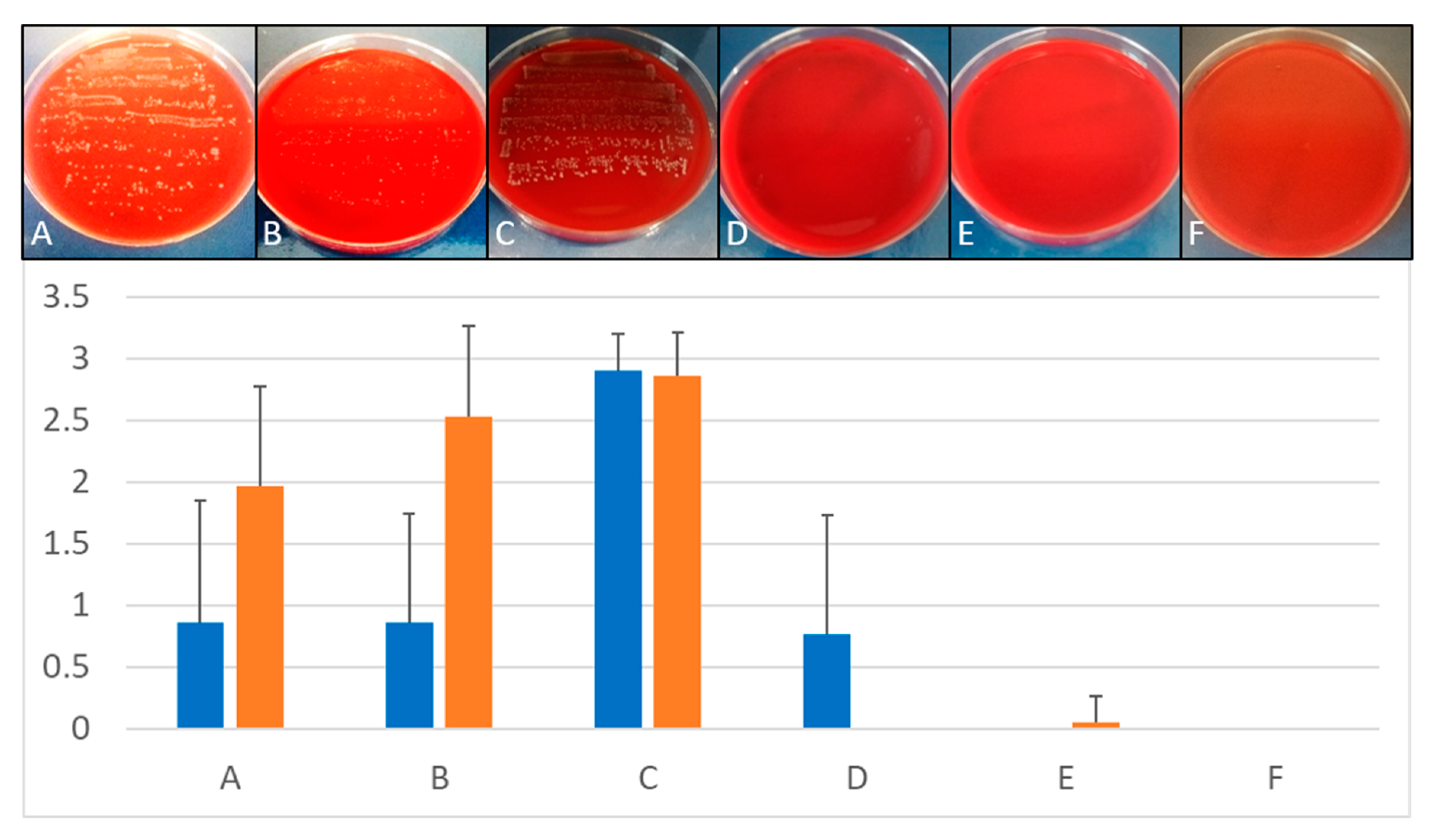
Figure viii. Treatment of multi-species biofilms. Upper console: Comparison of growth on Columbia Claret Agar plates subsequently different treatment of implants placed in bovine cadaver bone. Lower console: Quantitative comparison of growth on implants inserted in ribs depending on treatment protocols. (A) Mechanical debridement, (B) air abrasion, (C) BDD treatment for 0 min, (D) BDD treatment for v min, (E) BDD treatment for 10 min, (F) BDD treatment for 15 min (n = 3 for each methods and time point). Growth of implant roll-outs was rated from 0 (no growth) to 3 (strong growth). Columns stand for the mean of iii independent biological replicated ± standard deviation (SD).
Figure 8. Handling of multi-species biofilms. Upper console: Comparing of growth on Columbia Blood Agar plates after different treatment of implants placed in bovine cadaver bone. Lower panel: Quantitative comparison of growth on implants inserted in ribs depending on treatment protocols. (A) Mechanical debridement, (B) air abrasion, (C) BDD treatment for 0 min, (D) BDD treatment for 5 min, (E) BDD handling for 10 min, (F) BDD treatment for 15 min (n = 3 for each methods and time point). Growth of implant curlicue-outs was rated from 0 (no growth) to three (strong growth). Columns stand for the mean of 3 independent biological replicated ± standard difference (SD).
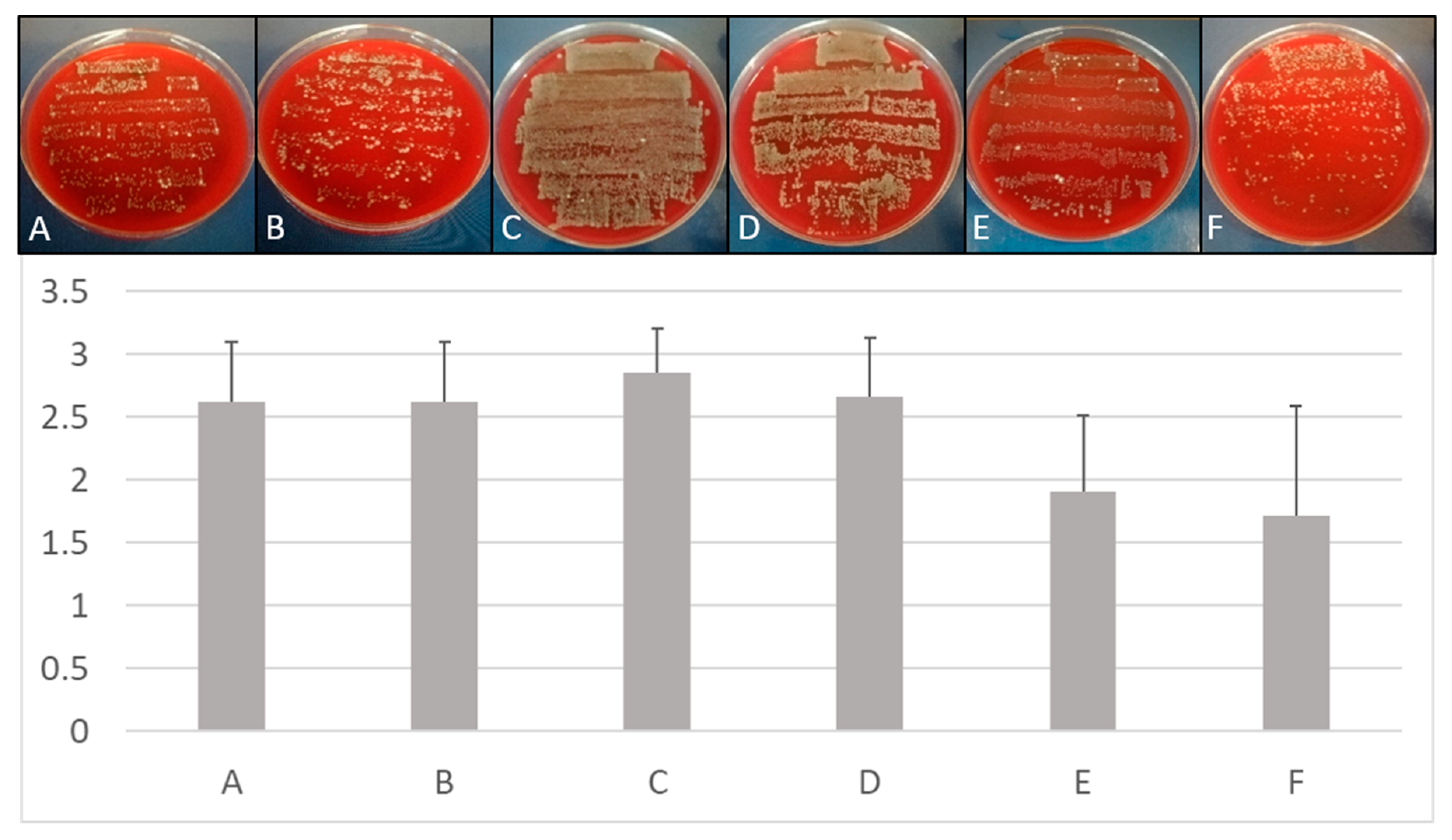
Figure ix. Influence of BDD electrode treatment on physico-chemical parameters. Changes in pH (upper console) and temperature (lower panel) during application of the BDD electrode in phosphate-buffered saline was tested. For each parameter, three contained experiments were carried out (n = 3, electric current 15–twenty mA; voltage 6 V).
Figure nine. Influence of BDD electrode treatment on physico-chemic parameters. Changes in pH (upper panel) and temperature (lower panel) during awarding of the BDD electrode in phosphate-buffered saline was tested. For each parameter, iii independent experiments were carried out (north = three, current 15–20 mA; voltage vi V).
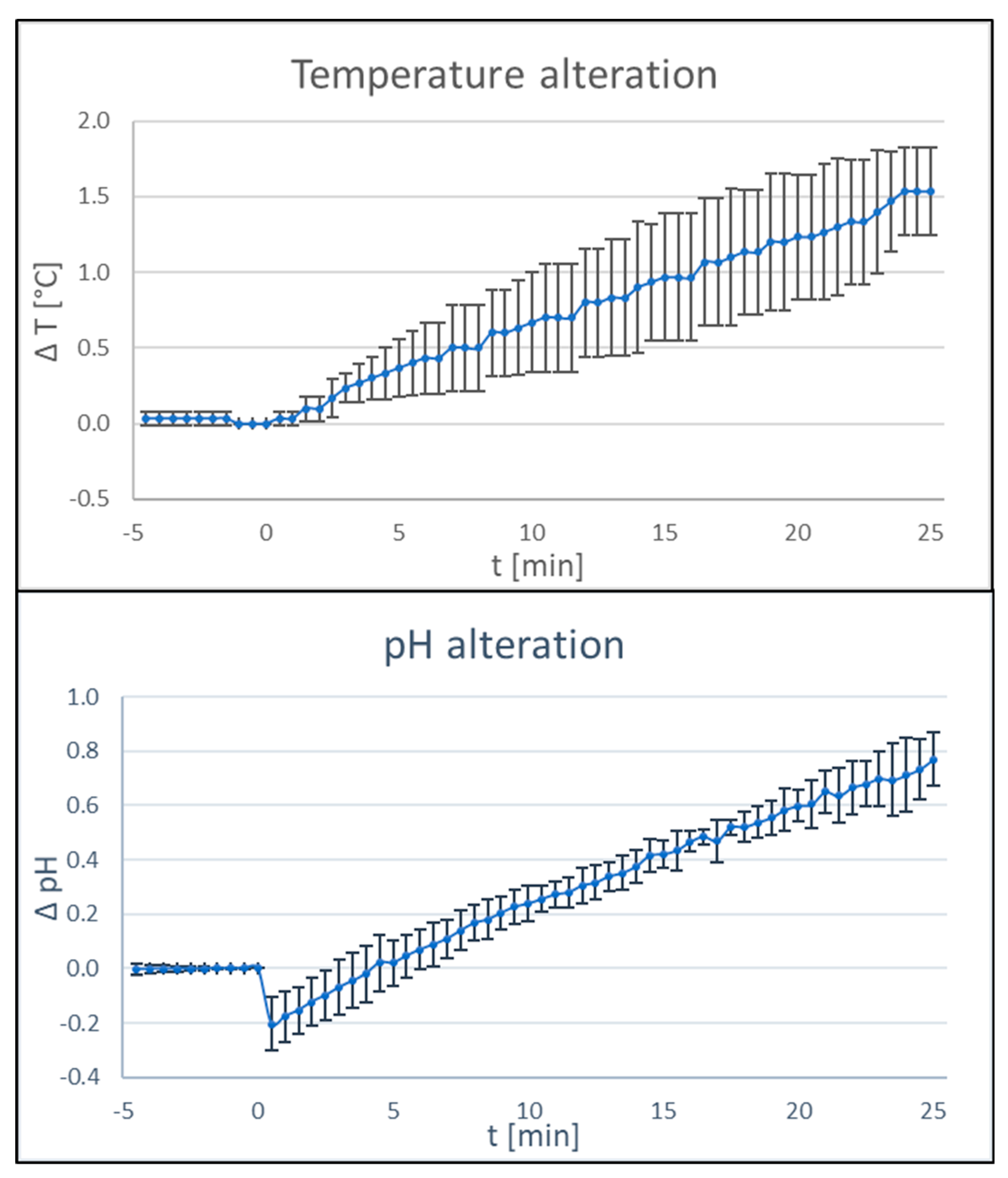
Table 1. Microorganisms used in this study.
Table 1. Microorganisms used in this written report.
| Species | Description | Reference/Source |
|---|---|---|
| Leaner | ||
| Bacillus pumilus | Gram-positive isolate from infected root culvert, spore erstwhile | [49] |
| Bacillus subtilis | Strain 168, Gram-positive spore former | Bacillus Genetic Stock Center (Columbus, OH, United states) |
| Enterococcus faecalis | DSM 20478, type strain, Gram-positive | High german Type Civilization Collection, DSMZ, Braunschweig, Germany |
| Roseomonas mucosa | Gram-negative isolate from infected root canal | [l] |
| Staphylococcus epidermidis | DSM 20044, type strain, Gram-positive | German Type Culture Collection, DSMZ, Braunschweig, Germany |
| Streptococcus sanguinis | DSM 20567, blazon strain, Gram-positive | German language Type Culture Collection, DSMZ, Braunschweig, Germany |
| Yeasts | ||
| Candida albicans | Strain SC5314 | Laboratory stock |
| Candida dubliniensis | Isolate from infected root canal | [50] |
Table 2. Mean values and standard deviations of ratings recorded for implants contaminated with E. faecalis, C. dubliniensis (implants placed in silicone or polyurethane foam) and multispecies biofilm (implants placed in bovine rib) post-obit 5 min of treatment with mechanical debridement, air abrasion and BDD electrode (n = 3 per microorganism and treatment method). Comparative statistics did not reveal whatever significant differences among the three modalities (p > 0.05).
Table 2. Mean values and standard deviations of ratings recorded for implants contaminated with E. faecalis, C. dubliniensis (implants placed in silicone or polyurethane foam) and multispecies biofilm (implants placed in bovine rib) following v min of treatment with mechanical debridement, air abrasion and BDD electrode (due north = three per microorganism and treatment method). Comparative statistics did non reveal any significant differences amidst the three modalities (p > 0.05).
| Microbial Species | Mechanical Debridement | Air Abrasion | Electrochemical Disinfection | Kruskal Wallis Chi-squared | df | p-Value | |||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||||
| Eastward. faecalis, polyurethane | 1.ninety | 0.83 | 2.fifty | 0.76 | 0.00 | 0.00 | 5.804 | 2 | 0.055 |
| East. faecalis, silicone | 0.86 | one.01 | 0.86 | 0.91 | 0.76 | 1.00 | 0.067 | 2 | 0.967 |
| C. dubliniensis, polyurethane | ii.07 | 1.39 | 1.33 | 0.82 | 1.53 | 0.52 | 0.605 | 2 | 0.739 |
| C. dubliniensis, silicone | 0.xl | 0.51 | 0.53 | 0.52 | 0.53 | 0.83 | 0.487 | ii | 0.784 |
| Multi-species biofilm, bovine rib | ii.62 | 0.50 | 2.62 | 0.fifty | ii.67 | 0.48 | 0.318 | 2 | 0.853 |
Table iii. Time for disinfection of implants placed in silicone depending on biofilm-forming species. All experiments were carried out in three contained biological replicates (northward = 3) and the longest necessary treatment time is given. Implants were pre-incubated with the respective microorganisms to permit biofilm formation for 3 to five days.
Table 3. Time for disinfection of implants placed in silicone depending on biofilm-forming species. All experiments were carried out in three contained biological replicates (due north = 3) and the longest necessary treatment time is given. Implants were pre-incubated with the respective microorganisms to allow biofilm formation for iii to 5 days.
| Species | Maximum Fourth dimension |
|---|---|
| Leaner | |
| B. pumilus | Incomplete disinfection within lx min |
| B. subtilis | Incomplete disinfection inside 60 min |
| Due east. faecalis | x min |
| R. mucosa | ten min |
| S. sanguinis | 10 min |
| South. epidermidis | 20 min |
| Yeasts | |
| C. albicans | 20 min |
| C. dubliniensis | 10 min |
| Mixture of microorganisms | |
| Multi-species natural biofilm developed on bovine ribs * | Incomplete disinfection within 15 min |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and atmospheric condition of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
stockdillgolou1940.blogspot.com
Source: https://www.mdpi.com/2077-0383/9/2/475/htm